4月25日-30日,备受关注的美国癌症研究协会(AACR)年会在美国芝加哥召开,本届会议以“统一癌症科学与医学:持续创新带来变革”为主题。秉持“疗效为核,百姓可及”的药物开发理念,上海细胞治疗集团持续推动JL-闪CAR-T™为核心的关键技术平台开发,实现原创非病毒载体基因写入技术、纳米抗体VHHMAb®技术等底层技术的持续突破,加速创新治疗药物开发进程。本次会议,集团共有5项最新研究成果获邀现场展示,覆盖从底层平台突破到优势抗体发现,再到临床应用转化的完整药物开发体系。充分展现了集团在革新CAR-T等创新细胞药物可及性和治愈性方面的前瞻布局及广阔前景。

针对传统CAR-T抗体发现的瓶颈,CARP平台(CAR-T AI-powered Rapid high-throughput screening Platform)为首个AI驱动、全谱系高通量的纳米抗体(VHH)筛选解决方案。由上海细胞治疗集团美国子公司Chantibody(CHANTIBODY THERAPEUTICS INC.)研发搭建。
上海细胞治疗集团与其美国子公司Chantibody(CHANTIBODY THERAPEUTICS INC.)合作开发的CARP平台,能够显著加速新型免疫疗法的发现与临床前评估,为CAR-T疗法的发展提供了精准、可扩展的新路径。未来,将进一步开展体内疗效和安全性验证,并拓展至更多肿瘤抗原和自身免疫疾病靶点。

摘要号:5889
摘要题目:
CARP: A High-Throughput Platform for Rapid VHH Discovery of Potent CAR-T Therapy
摘要内容:
传统CAR-T筛选包括:先筛选特异性结合目标抗原的抗体,再将抗体作为胞外抗原识别区制备嵌合抗原受体,然后体外验证效果。然而,传统CAR-T筛选面临显著的瓶颈问题:1. 抗体筛选速度慢,2. 难以高通量识别具有多样表位覆盖的、高质量抗体, 3. 往往因表位覆盖不全导致功能漏检或误选。这些因素共同导致了CAR-T研发周期延长,资源消耗加剧,并且可能错失真正的具有临床价值的分子。
VHH以其分子量小、可模块化拼接的优势,为构建双/多特异性CAR-T提供了理想支撑,但要实现高通量、全谱系的功能化筛选,仍需新一代技术平台。我们基于CAR-T筛选的痛点搭建了CARP高通量筛选平台。CARP平台在短时间内有效识别并聚焦潜在高亲和力、高特异性VHH。本次研究基于CARP对CDH17(Cadherin-17)蛋白免疫羊驼与美洲驼所得的VHH进行高通量筛选,加速获得了优化的VHH CAR-T(图1)。
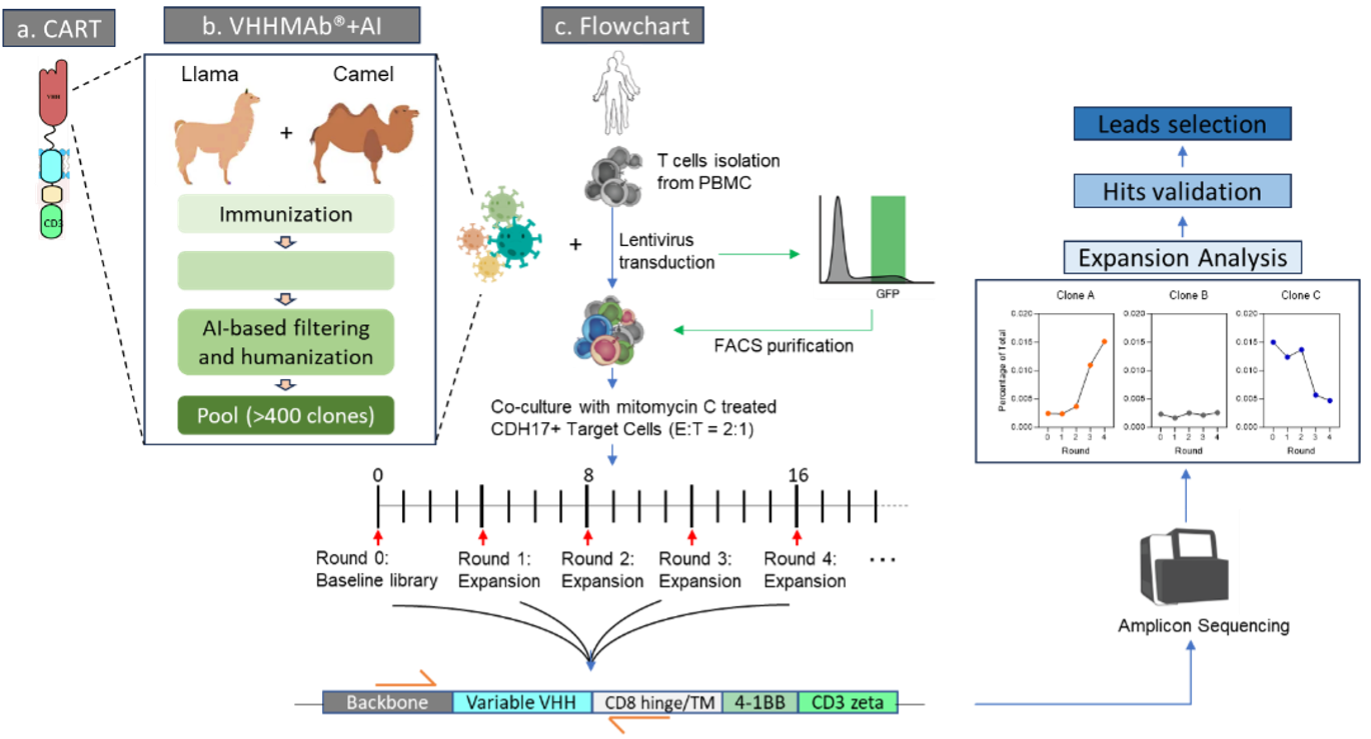 Figure 1. CARP high throughput screening workflow: It begins hits discovery by using VHHMAb® platform, followed by AI-powered VHH library construction that encompasses antibodies with diverse epitopes and low immunogenicity. This is followed by pooling CAR-T clones, co-culturing with target cells, performing NGS of amplicons from the amplified CAR-T clones, and concluding with lead validation.
Figure 1. CARP high throughput screening workflow: It begins hits discovery by using VHHMAb® platform, followed by AI-powered VHH library construction that encompasses antibodies with diverse epitopes and low immunogenicity. This is followed by pooling CAR-T clones, co-culturing with target cells, performing NGS of amplicons from the amplified CAR-T clones, and concluding with lead validation.
在完成VHHMAb®技术平台CDH17 VHH谱系分析并且建库后,获得>400具备良好的人源化,免疫原性的VHH。并进行了多细胞模型共培养验证CART的目标依赖性的增殖能力。在高表达CDH17的H716细胞、中等表达的Colo205细胞与CDH17阴性的HCT-116细胞中,CAR-T的扩增表现出显著差异。H716共培养组CAR-T阳性率及倍增幅度最高,Colo205组中等,HCT-116组则基本无增殖(图2)。该结果直观证明了候选VHH在不同表达水平肿瘤细胞中的目标依赖性扩增能力。
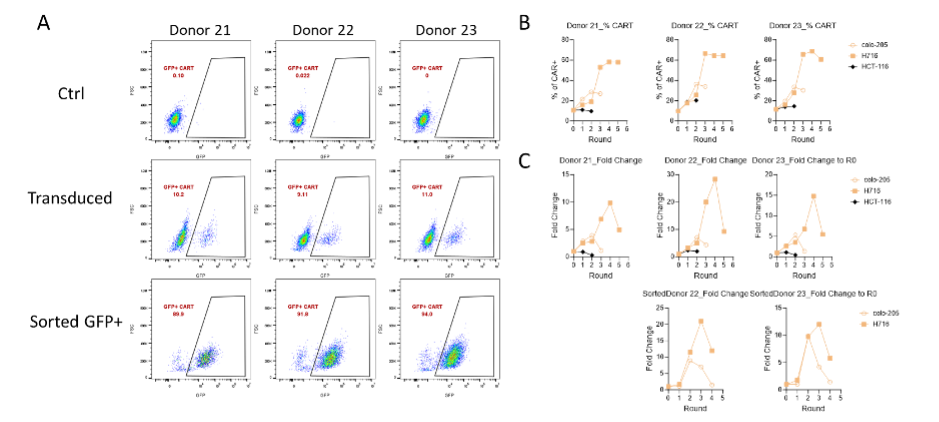
Figure 2. The preparation of pooled CAR-T and serial proliferation by co-culturing with target+ cell lines. After CAR-T preparation (A), the percentage (B) and fold change (C) of CAR-T proliferation were assessed after co-culturing with H716 (CDH17 high), Colo205 (CDH17 medium), and HCT-116 (CDH17 negative) cells.
NGS测序及增殖能力排序,通过对筛选池中每轮共培养后细胞群体进行NGS测序,获取各克隆的相对丰度变化曲线,并用斜率模型进行拟合(图3A、3B),实现对数百候选的快速定量排名;随后利用层级聚类(图3C)分层筛选出高潜力(快速斜率上升)与低潜力(曲线平缓或下降)VHH群体。
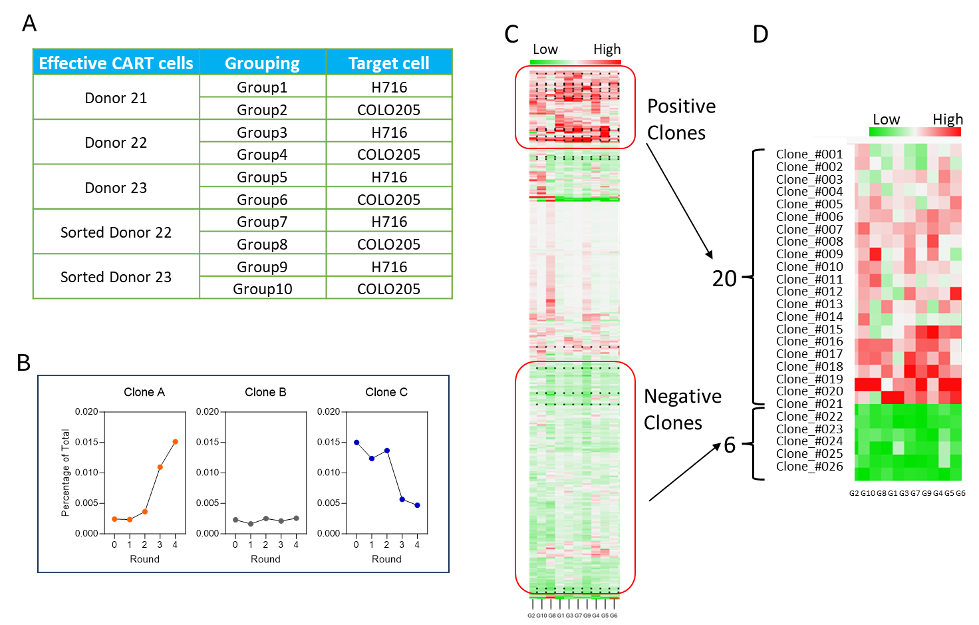 Figure 3. NGS and slope analysis for VHH hit ranking. (A) Grouping information. (B) The proliferation curve shows the clone percentage of each round based on NGS, with the slope of the curve calculated through fitting. Three examples are shown to demonstrate different types of slopes. (C) Hierarchical clustering analysis for lead selection. (D) Twenty positive and six negative clones were selected for validation.
Figure 3. NGS and slope analysis for VHH hit ranking. (A) Grouping information. (B) The proliferation curve shows the clone percentage of each round based on NGS, with the slope of the curve calculated through fitting. Three examples are shown to demonstrate different types of slopes. (C) Hierarchical clustering analysis for lead selection. (D) Twenty positive and six negative clones were selected for validation.
通过验证筛选结果发现CARP具备高准确率。从NGS及斜率排序中挑选20个高潜力克隆和6个低潜力克隆进行功能验证。高潜力组:19/20个克隆在H716共培养中表现出强劲扩增(图4A);低潜力组:5/6个克隆几乎无扩增(图4B);传统筛选对照:仅2/5个常规亲和力优选克隆表现出良好扩增(图4C)。该验证体系精准区分出功能强弱,证明CARP在候选VHH筛选上的高效与可靠。
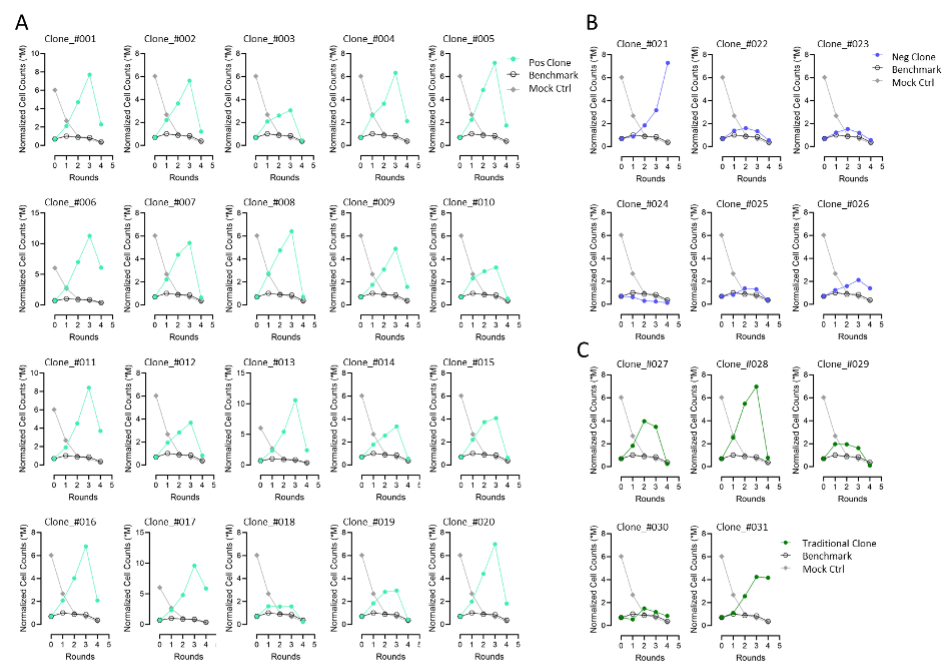
Figure 4. Leads validation to confirm CARP accuracy in lead selection. Selected CART leads were co-culturing with H716 cells. (A) In the group of positive clones, 19 out of 20 demonstrated strong CAR-T expansion. (B) In the group of negative clones, 5 out of 6 showed no or minimal CART expansion. (C) For comparison, conventional CAR-T leads (selected based on good binding affinity to proteins and cells) were tested, and only 2 out of 5 exhibited strong CAR-T expansion.
结论:
1. 高准确率命中:20个功能阳性克隆中有19个在后续功能实验中一致表现优异;6个阴性克隆中5个未能增殖,验证了CARP平台的筛选精度。
2. 优质VHH候选:成功识别多个靶向CDH17同一结构域的VHH, CAR-T的扩增与肿瘤细胞杀伤活性均优于临床对照与传统筛选分子。
3. 加速开发路径:平台一次免疫、一次筛选即可完成人源化和功能评估,大幅缩短新型CAR-T疗法的前期研发周期。
